
Contact our team
Need Help, Contact us
Global

Merz Therapeutics’ R&D and production activities are the foundations of our leadership in specialty neurology.
We work tirelessly to develop new treatments through in-house clinical development and supporting the research of others. Meanwhile we work constantly to grow the indications and availability of our existing portfolio.
Our research facility in Frankfurt, Germany, accommodates 150 colleagues. In addition, we operate four production sites – two internal and two external. These sites bring together colleagues from all around the globe, all working closely to discover, develop, and deliver therapeutics for specialty neurology conditions.
Our team contains world-class expertise across multiple essential disciplines, including:
R&D is also responsible for managing over 800 product licenses in over 80 countries.

Pre-clinical sciences and toxicology
Botulinum Neurotoxin biotechnology and formulation development
Clinical sciences and operations, biometrics, contract research organization management

Clinical trial supply
(Phase II-IV)

Global regulatory affairs

Global product safety / pharmacovigilance

Project management

Good practice quality assurance
Life cycle management / product maintenance
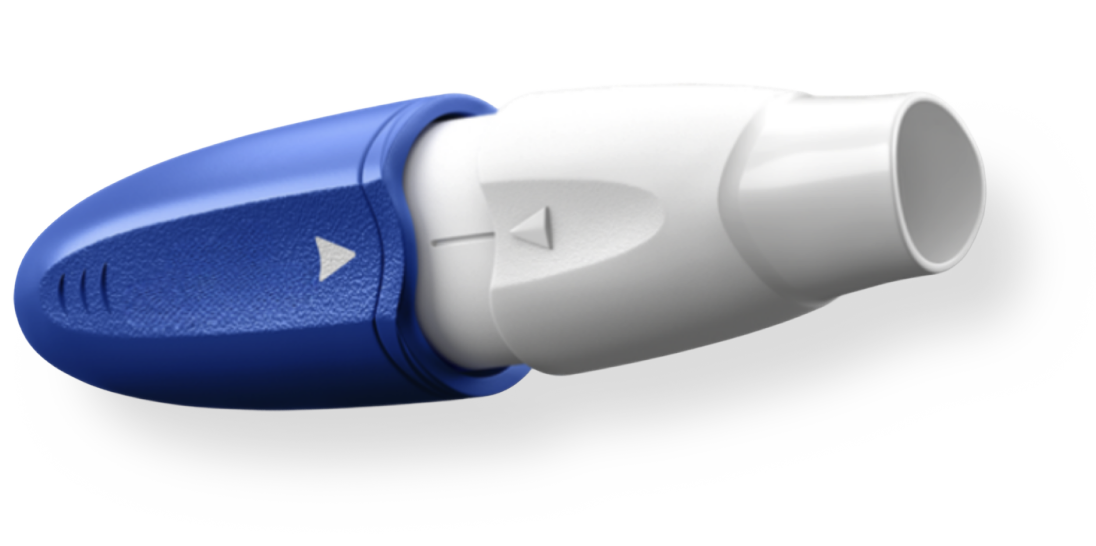
At Merz Therapeutics, we are committed to innovation by unlocking the full potential of established medicines for the good of patients. One example of this is the inhaled administration of levodopa using our patented Arcus® delivery system. When taken orally, levodopa absorption can vary greatly due to factors such as the timing of meals and the type of food consumed. Our inhaler for levodopa avoids this variability, making on-demand use a possibility for patients who need it.
Merz Therapeutics has developed XTRACTTM, a unique manufacturing process for botulinum toxin that utilizes chromatography to remove complexing proteins, thereby leaving only the active therapeutic product and minimizing waning efficacy due to immunogenicity.
Our Medical Affairs work is a bridge between patients, physicians and us. We are committed to healthcare provision “beyond the product”.
Merz Therapeutics provides clear and current medical information and education for all our products, and keeps that information aligned to customers’ changing needs.
We constantly ask our customers and patients what they need, and adapt our programs in response.
In 2023-2024 we:
Includes:
You are leaving this website. With respect to the content of the following page, as well as to links to other websites located on this page, Merz Therapeutics GmbH has no way of controlling the content of these sites. Merz Therapeutics GmbH assumes no responsibility for the content of these sites or the consequences of their use by visitors. However, we ask you to notify us immediately of any illegal content on the linked sites.
You are leaving this website. The content of the following sites maintained by the parent company or another affiliated company, or links to other sites located on this site, is subject to the legal requirements of the country in which the site is maintained. Merz Therapeutics GmbH accepts no responsibility whatsoever for the content of these websites or for the consequences of their use by visitors. However, we ask you to notify us immediately of any illegal content on the linked sites.